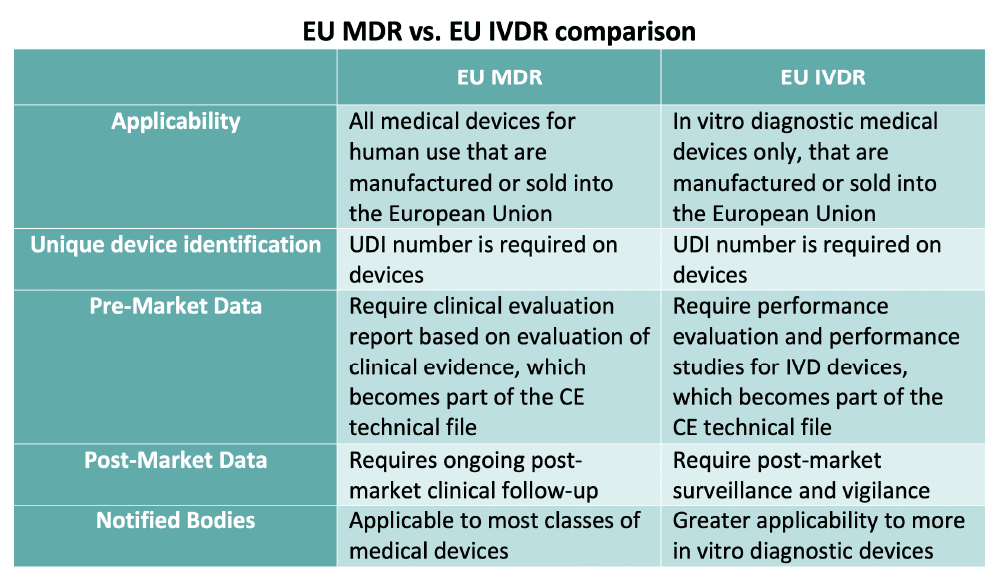
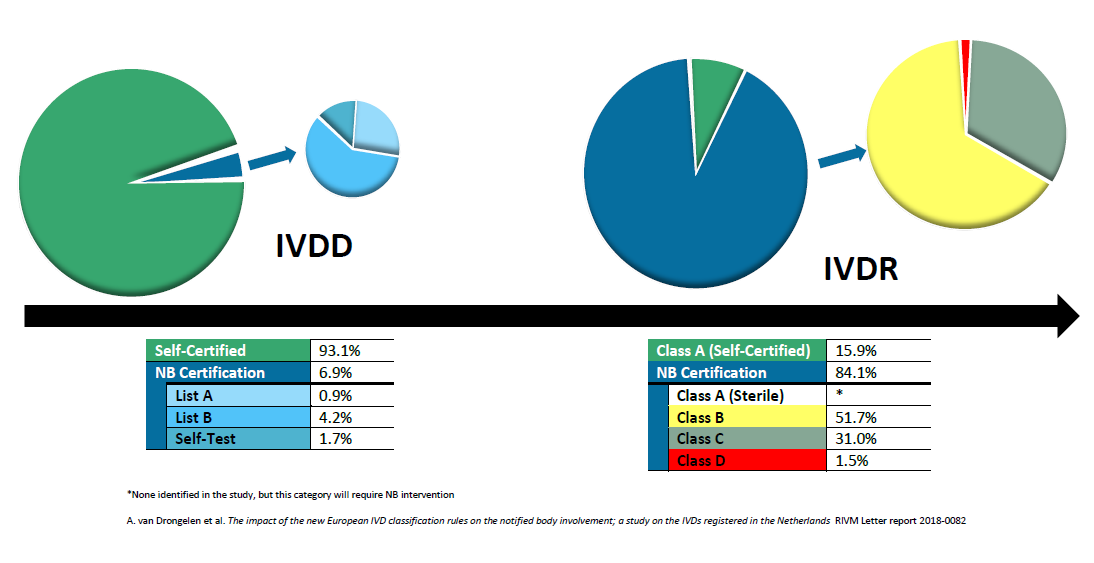
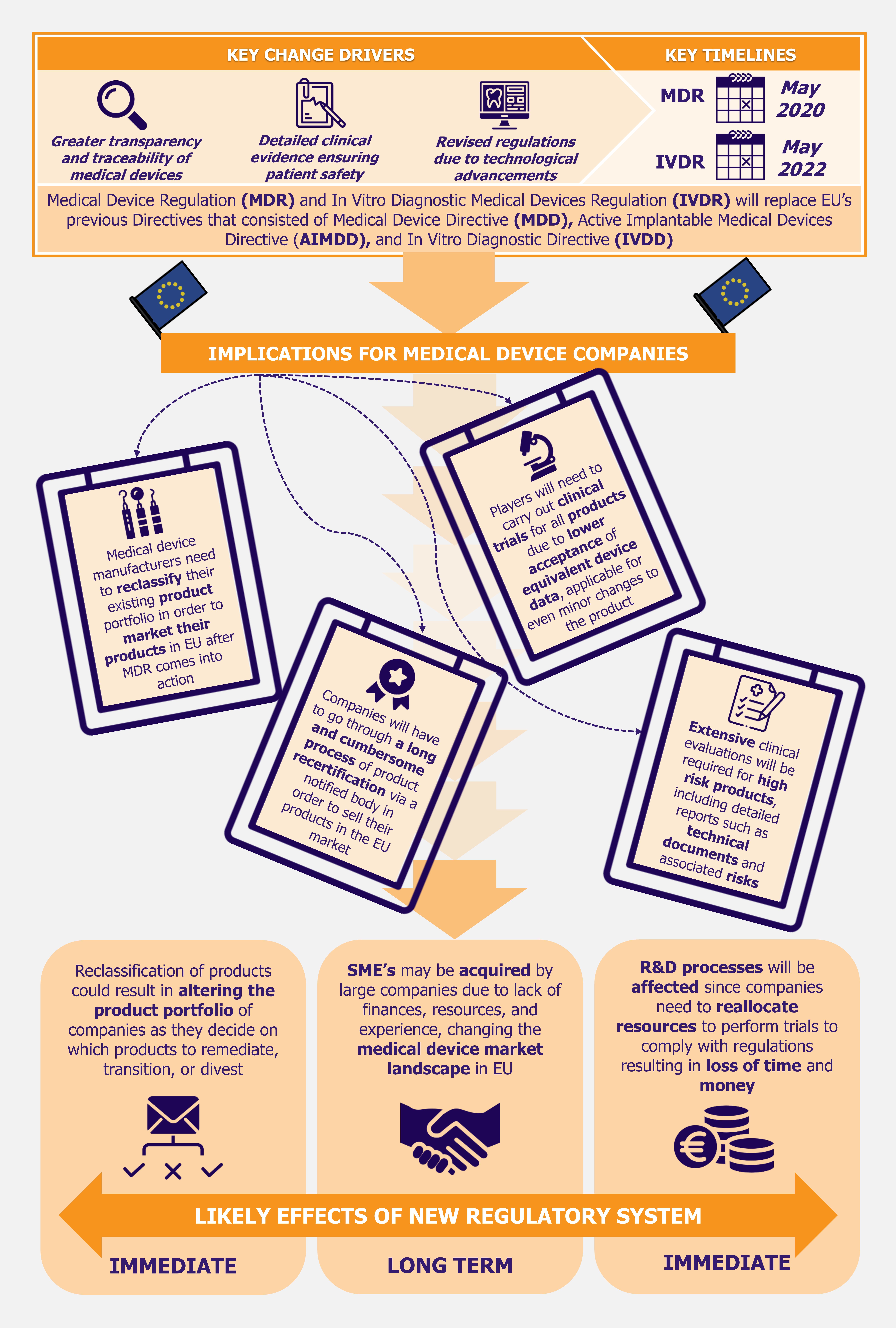
The EU's Medical Device Regulation (EU) 2017/745 – Are You Ready for Huge Sweeping Changes? - In Compliance Magazine
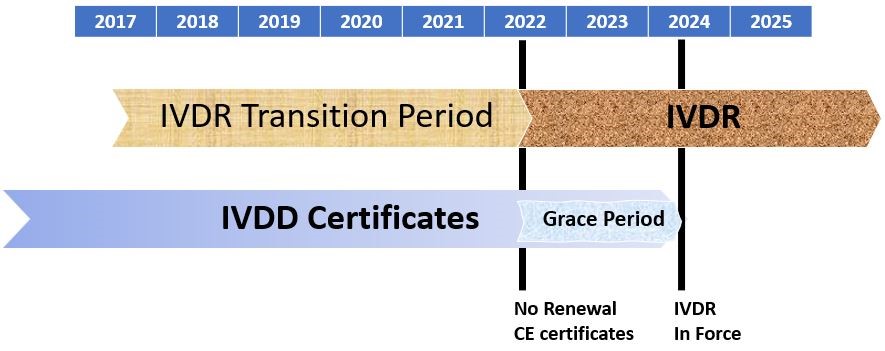
Key Changes in the Regulatory Requirements for In Vitro Diagnostic Devices Marketed in the European Union Under IVDR 2017/746 - Criterion Edge